Well three times now we have been ready to watch for the rocket launch only to have it scrubbed. So, what to do? Let's review some recent observations using gamma rays to get star formation history.
First though we followed the live streaming of the launch sequence as we set up the camera and tripod. We had a great location and the clouds were mostly not present. The live stream video had great images of the launch site and you could even see outgassing of vapors from various locations on the rocket. It was fueled up and ready to go. Then we could here mission control getting advisories about high hydrogen levels in one of the engine areas. Finally, we heard the discussion from the teams investigating if the problem was significant or not and yes, it turned out the launch was going to be scrubbed.
 |
| Live streaming on iPhone of Delta IV Heavy launch site, just before scrub announcement (Source: ULA) |
Darn! Well, the camera and tripod were all set up, so we had to take at least one picture and the nearly full moon was an easy consolation target. Now we have to wait again and see if the next launch window is more successful!
 |
| Ok, the launch is scrubbed, but the moon is up. DSLR, 300mm, 1/250 second (Source: Palmia Observatory) |
Ok, so now we get to use our cancelled observation time to read and report on an interesting article just published (Nov 30, 2018) in Science magazine on measuring gamma ray interaction with the starlight. The high energy gamma rays are not produced by nuclear reactions inside of most (90%) stars, but instead originate mostly from sources like very distant high energy quasars. When these gamma rays interact with the available starlight, the energy flux of the gamma rays is decreased slightly and this dip in intensity can be measured. Then with this dip and the measured red shift of the gamma rays, it is possible to estimate the amount of star formation based on the amount of starlight present at that redshift. The cartoon below illustrates this concept. Pretty neat!
 | |
|
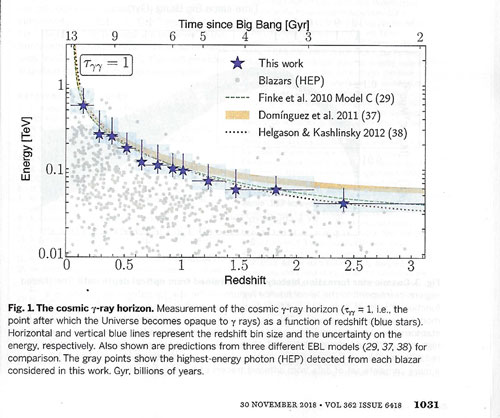
If you are interested in more of the details, I have included two more charts showing the measured data. The chart below shows the measured energy levels, associated redshifts, where the universe becomes opaque to gamma rays.
 |
| Using gamma rays to estimate star formation history (Source: LAT Collaboration, Science, 30 Nov 2018) |
In this chart, the peak star formation period is seen to be right around redshift 2, which corresponds to a time period of about 3-4 billion years after the big bang.
 | |
|
On a slightly different energy level from gamma rays, we can also take a look at the sources of ultraviolet and visible light that is produced by stars and other sources and is more accessible to many amateurs and other astronomers.
All of these lines are caused by atomic transitions between various quantum energy levels. The Lyman series is the highest energy hydrogen transition lines and are found in the ultraviolet region. The Lyman Alpha ultraviolet line at 121.6 nm, has been very instrumental in helping determine the structure of the universe. The first Balmer line, also called the Hydrogen Alpha line, is in the red visible spectrum
 |
| Energy level transitions generates ultraviolet and visible spectrum (Source: D. Griffiths, Intro to Quantum Mechanics) |
In the early days in 1913, Niels Bohr developed the theory that could explain these spectral lines. Now you probably have read various quantum mechanics textbooks and seen these diagrams before, but the very discrete nature of these atomic transitions was not always apparent to those studying them at the time. For instance, I just barely read a couple of chapters out of Ballentine's book: Quantum Mechanics, A Modern Development, where the author describes how these atomic transitions were observed, not a visual spectral lines, but just different levels of intensity of ionization current, for example.
Check out the graph below, generated in 1914, where the atomic transitions in mercury. As the ionization voltage in the lab setup is increased the various quantum energy transitions start to show up. But in this low resolution measurement it is not apparent that the energy transitions are actually very distinct and sharp. These early measurements seemed to indicate sort of a continuous process and not the discrete quantum mechanical reality and it was not until higher resolution measurements were completed that it became more obvious about the discrete nature of reality.
 |
| Early 1914 example of low resolution quantum jumps for mercury (Source: L. Ballentine, "Quantum Mechanics") |
Then, from the same textbook, we find another example of very high resolution measurement of transitions in hydrogen. These transitions are not from like primary quantum number, n, values of 2 back to 1 or from 3 back to 2, but very high quantum n numbers up in the 60-70 range. I have plugged different values of n into Bohr's equation for low values of n equal to say 2 or 3, but found this very interesting and had no idea that transitions up this high had been made. As the energy of the system is increased and n keeps increasing and increasing, then eventually the atom is completely ionized and the electron escapes from the atom entirely. Of course, that is what the theory of the hydrogen atom predicts, but to see this measurement was very enlightening for this senior student.
 | |
|
Ok, enough astrophysics for now and we are free to schedule the next launch observing session now apparently scheduled for Thursday, December 30. Ok, we have put the date on the observing calendar and hopefully this time, if the rocket hydrogen leak can be fixed, we will have a successful launch and some clear skies to see the rocket plume high up in the atmosphere!
Until next time,
Resident Astronomer George
If you are interested in things astronomical or in astrophysics and cosmology
Check out other postings on this blog at www.palmiaobservatory.com

No comments:
Post a Comment